Air pollutants are emitted and formed both outdoors and indoors, resulting in personal pollutant exposures that can greatly exceed levels measured by routine air measurements made at central-site ambient outdoor air monitoring stations. Pollutants can be usefully categorized into three classes: (1) pollutants primarily emitted into the outdoor environment, (2) pollutants primarily emitted into the indoor environment, and, (3) pollutants emitted into both outdoor and indoor environments, as summarized in Table 1. The primarily outdoor air pollutants, and their typical sources, are discussed in greater detail in this research paper.
Sulfur Oxides
Sulfur oxides are present in the ambient air primarily as primary gaseous SO2 or secondary particulate sulfate(SO42-). SO2 is formed when fossil fuels containing sulfur (mainly coal or oil) are burned, and by metal smelting and other industrial processes. The highest monitored concentrations of SO2 are usually recorded in the vicinity of large industrial facilities lacking modern emission controls, such as older coal-fired power plants. In the United States, fuel combustion accounts for most of the total SO2 emissions (U.S. EPA, 2000a). Over 65% of SO2 released to the air in the United States comes from electric utilities, primarily from units that burn coal. Other sources of SO2 are industrial facilities that derive their products from raw materials like metallic ore, coal, and crude oil, or that burn coal or oil to produce process heat. Examples include petroleum refineries, cement manufacturing, and metal processing facilities. Also, locomotives, large ships docked at deep ports (which often burn especially polluting ‘bunker’ fuel), and some non-road diesel equipment still burn high sulfur fuel and release SO2 emissions to the air in large quantities. Natural sources of SO2 include releases from volcanoes, biological decay, and forest fires.
SO2 is also a major source of secondary PM air pollution. Once emitted into the air, SO2 can dissolve in water vapor to form acidic aerosols (by heterogeneous formation), and can also interact with other gases and particles in the air to form sulfates and other PM products via homogeneous formation. These sulfate particles can be harmful to humans and to their environment, including via acid rain. Secondary fine particle sulfates formed in the atmosphere from SO2 unfortunately also have a typical diameter roughly equal to that of the optimum visible wavelength of sunlight (0.5 mm), so they can also cause significant visibility impairment via the scattering of light, most noticeably as a milky white haze during afternoons in the summer months. In addition, their small size allows these sulfates to be transported over long distances, far from the point of original SO2 emission. Thus, air pollution problems resulting from sulfur oxide emissions are not limited to the localities where these pollutants are first emitted.
Ozone
Ozone (O3) is a secondary gas that is not usually emitted directly into the air, but is instead created in the atmosphere by a chemical reaction between oxides of nitrogen (NOx) and volatile organic compounds (VOCs) in the presence of sunlight. Ozone has the same chemical structure whether it occurs miles above the earth or at ground level and can be deemed ‘good’ or ‘bad,’ depending on its location in the atmosphere. ‘Good’ ozone forms naturally in the stratosphere, approximately 20 miles above the Earth’s surface, forming a protective layer around the globe that absorbs the sun’s most harmful ultraviolet rays. At the surface, however, the presence of ozone is largely not natural, and can result in adverse human health effects when breathed.
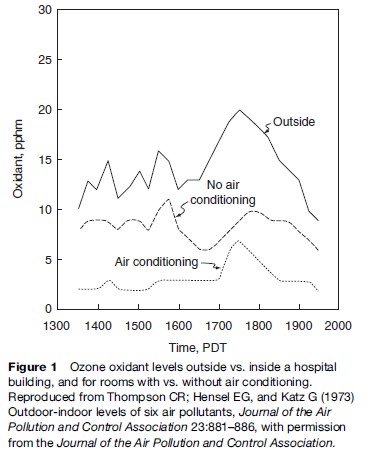
Motor vehicle exhaust, industrial emissions, gasoline vapors, and chemical solvents, as well as some natural sources (such as vegetative matter), emit VOCs into the atmosphere, which can then react with NOx via a complex series of atmospheric reactions to form O3 in the presence of warmth and sunlight. Many urban areas therefore have high levels of ozone during the warmer months (with more hours of sunlight), but even rural areas are also exposed to increased summertime ozone levels because winds can carry ozone (and the precursor pollutants that form it) hundreds of miles away from their original source locale. As a result, O3 is usually a summertime air pollutant, with concentrations commonly peaking in the late afternoon, as shown in Figure 1. As also shown in Figure 1, levels of ozone diminish indoors, as it is primarily formed outdoors, and upon entering a building quickly reacts with the interior walls and furnishings and is removed from the air, especially when air conditioning is present in the building.
Particulate Matter
Particulate matter (PM) represents a broad class of chemically and physically diverse aerosols composed of solid particles or liquid droplets suspended in the air. Such aerosols can be characterized by their size, formation mechanism, origin, chemical composition, atmospheric behavior, and method of measurement. PM air pollution can be viewed in two major components: primary PM, or soot, emitted directly into the atmosphere by pollution sources such as industry, electric power plants, diesel buses, and automobiles, and secondary PM, formed in the atmosphere from primary gaseous pollutants, such as sulfur dioxide and nitrogen oxide gases. The concentration of particles in the air varies across space and time and is related to the source of the particles and the pollutant transformations that occur in the atmosphere. In many parts of the developing world, cooking and heating are done by using wood, crop waste, or dried animal dung as fuel (e.g., see Smith et al., 2004), causing even greater exposures to PM, both outdoors and indoors.
PM air pollution is commonly characterized as particles that fit the following general size fractions:
- PM10 (defined as all particles equal to and less than 10 micrometers (mm) in aerodynamic diameter). Particles larger than this are not generally able to be breathed past the trachea, and are caught in the nose and throat, not depositing in the lung.
- PM10–2.5, also known as coarse fraction particles (defined as those particles with an aerodynamic diameter greater than 2.5 mm, but equal to or less than a 10 mm in diameter).
- 5, also known as fine fraction particles (generally defined as those particles with an aerodynamic diameter of 2.5 mm or less), can be breathed into the deepest recess of the lung.
- Ultrafine particles, generally defined as those less than 0.1 mm in diameter. Some scientists have hypothesized that ultrafine particles, because of their small size and large surface area to mass ratio, may be especially toxic.
Fine and coarse particles differ in terms of the emission sources, formation processes, chemical composition, atmospheric residence times, transport distances, and other parameters. Fine particles are directly emitted from combustion sources, and are also formed secondarily from gaseous precursors such as sulfur dioxide, nitrogen oxides, or organic compounds. During the summer months, these secondary particles often dominate the PM2.5 mass. Such fine particles are commonly composed of sulfate, nitrate, chloride, and ammonium compounds, organic and elemental carbon, and metals. Combustion of coal, oil, diesel, gasoline, and wood, as well as industrial process sources (such as smelters and steel mills), can produce emissions that contribute to ambient fine particle concentrations. Fine particles can remain in the atmosphere for days to weeks and travel through the atmosphere hundreds to thousands of kilometers, whereas most coarse particles typically deposit to the Earth within minutes to hours and within tens of kilometers from the emission source.
Coarse particles are typically mechanically generated outdoors by crushing or grinding operations, but atmospheric concentrations are often dominated by dusts and crustal material resuspended by traffic on paved or unpaved roads. Other outdoor sources of coarse particles include those from construction, farming, and mining activities.
Nitrogen Oxides
Nitrogen oxide (NOx) pollution is formed whenever fuel is combusted at high temperatures by a ‘fixing’ of the nitrogen in the combustion chamber’s dilution air into NOx. Many of the nitrogen oxides are colorless and odorless. However, one common NOx, nitrogen dioxide (NO2), along with particles in the air, can often be seen as a reddish-brown layer in the air over urban areas. NOx is one of the main ingredients involved in the formation of ground-level ozone, which can trigger serious respiratory problems. It also reacts to form nitrate particles and acidic aerosols, contributing to the formation of acid rain. Particulate nitrates resulting from NOx contribute to fine atmospheric particles that can cause visibility impairment. NOx gases also contribute to the global warming problem.
The major outdoor sources of NOx are primarily emissions from transportation (virtually all of which are from motor vehicles) and fuel combustion. In the United States, transportation emissions account for over half of all NOx emissions. The other half of U.S. outdoor NOx emissions is largely a result of fuel combustion emissions, which are roughly evenly split between electric utilities (at 22%) and all other industrial, commercial, and residential combustion (at 20%) (U.S. EPA, 2000b). Natural processes can also form NOx, including volcanoes, oceans, biological decay, and lightning strikes. Indoor sources of NOx include kerosene heaters, unvented gas stoves and heaters, and cigarette smoking.
Volatile Organic Compounds
Volatile organic compounds (VOCs) are organic chemicals that readily produce vapors at ambient temperatures, and are therefore emitted as gases from certain solids or liquids. All organic compounds contain carbon, and organic chemicals are the basic chemicals found in all living things. Many organic compounds in our air today do not occur naturally but are instead man-made, formed during industrial processes or combustion. VOCs include a variety of chemicals, some of which may have short and long-term adverse health effects. Sources of VOCs include gasoline, industrial chemicals such as benzene, solvents such as toluene and xylene, and perchloroethylene (principal dry cleaning solvent). VOCs are also released from burning fuel, such as gasoline, wood, coal, natural gas, and from solvents, paints, glues, and other products used at home or work. Many VOCs are also classified by the U.S. EPA as ‘air toxics.’ Toxic air pollutants are defined as those pollutants that cause or may cause cancer or other serious health effects, such as reproductive effects or birth defects. In the United States, industrial processes (at 47%) and vehicle emissions (at 44%) are the dominant sources of outdoor VOCs (U.S. EPA, 2000b).
Carbon Monoxide
Carbon monoxide, or CO, is a colorless, odorless gas formed when carbon in fuel is not completely combusted. CO is toxic to humans because it very effectively competes with oxygen in the blood for binding sites with the heme portion of blood cells, reducing the ability of the blood to carry oxygen to various parts of the body, including the heart and brain. When breathed at acutely high levels, CO can be fatal, and indoor exposures to fire pollution and emissions from faulty appliances indoors causes thousands of deaths per year in the United States (Kleinman, 2000).
CO is a component of motor vehicle exhaust, which contributes most of the emission of outdoor CO. In the United States, a total of about 56% of all outdoor CO emissions nationwide result from on-road vehicles (U.S. EPA, 2000b). Off-road engines and vehicles (such as construction equipment and boats) contribute another 22% of all CO emissions nationwide, or nearly one-third of all vehicular emissions of CO. Thus, higher levels of CO generally occur in areas with heavy traffic congestion, such as near entrances to tunnels. In cities, nearly all CO emissions may come from motor vehicle exhaust. Other outdoor sources of CO emissions include industrial processes (such as metals processing and chemical manufacturing), residential wood burning, and natural sources such as forest fires. Woodstoves, gas stoves, cigarette smoke, and unvented gas and kerosene space heaters are sources of CO indoors. The highest levels of CO in the outside air typically occur during the colder months of the year, when, as is discussed in the next section, atmospheric inversion conditions are more frequent, thus leading to higher localized pollution levels.
Carbon Dioxide
Carbon dioxide (CO2) is emitted as a result of fossil fuel combustion (e.g., burning of coal, oil, and natural gas). At high concentrations, CO2 can also have direct human health effects and can cause burns, frostbite, and blindness if an area is exposed to it in solid or liquid form. If inhaled, it can be toxic in high concentrations, causing an increase in breathing rate, unconsciousness, and death. But its greatest importance is as a component of the Earth’s atmosphere. CO2 is only a minor constituent of natural air (about 0.03%), but the increased use of fossil fuels is increasing the amount of carbon dioxide in the atmosphere. This is a problem because CO2 is a principal ‘greenhouse effect’ gas. The greenhouse effect is the heating of the Earth due to the presence of gases in the atmosphere that cause an effect similar to that produced by the glass panes of a greenhouse: shorter-wavelength solar radiation from the sun passes through Earth’s atmosphere, but when part of the energy absorbed by the Earth is then reradiated back to the atmosphere as long-wave infra-red radiation, the greenhouse gases capture the long wave infrared light, causing the lower atmosphere to warm. This ‘greenhouse gas’ process is a natural one that has been happening on Earth for millions of years but is now being greatly increased by human emissions of greenhouse gases such as CO2.
Lead
The major sources of atmospheric lead (Pb) emissions have historically been motor vehicles (such as cars and trucks) and industrial sources. However, leaded gasoline was phased out of use in the United States during the mid-1970s through mid 1980s, and in Europe during the 1990s. Since the phase-out of leaded gasoline in the United States, metals processing has become the major source of lead emissions to the outdoor air, representing some 52% of all emissions in the United States, with all industrial processes accounting for some 74% of Pb emissions (U.S. EPA, 2000b). Virtually all remaining transportation emissions of Pb in the United States are now by off-road vehicles. Other stationary sources of lead include waste incinerators and disposal (accounting for some 16% of the total), utilities, and lead-acid battery manufacturers (U.S. EPA, 2000b). In certain countries of the developing world, lead has yet to be phased out of all gasoline, so automotive emissions remain a significant concern in those countries. Progress continues on controlling lead in the environment, however, and, as of the end of 2005, the sub-Saharan nations of Africa phased out use of leaded gasoline in automobiles (Washington Post , 2006).