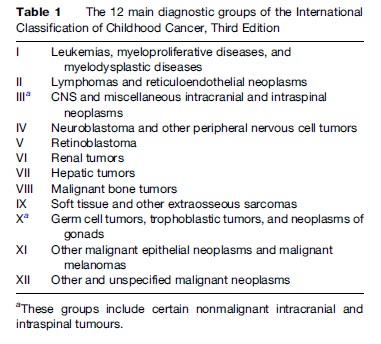
Cancer incidence data for people of all ages are usually presented according to the International Classification of Diseases (ICD). In the current, tenth revision of ICD, cancers other than leukemias, lymphomas, Kaposi sarcoma, cutaneous melanoma, and mesothelioma are classified solely by primary site. For the remaining cancers in adults, the great majority of which are carcinomas, this is reasonably satisfactory. Childhood cancers, however, are histologically much more diverse and include several distinctive types that arise in embryonal precursor cells and are exceedingly rare in later life. It has long been recognized that childhood cancers are more appropriately classified according to morphology as well as site. Standard classifications have been devised with groups defined according to their codes in successive editions of the International Classification of Diseases for Oncology (ICD-O). The most recent is the International Classification of Childhood Cancer, Third edition (ICCC-3). It was designed to agree with current international standards, notably the WHO Classification of Tumours, to include all morphology codes in the Third Edition of ICD-O and to provide continuity with previous classifications. ICCC-3 contains 12 main diagnostic groups (Table 1). These are split into 47 subgroups, and the most heterogeneous subgroups are each split into between two and 11 divisions. The nomenclature of many diagnostic categories is new to ICCC-3, but the contents of the main groups and most of the subgroups are very similar to those in its predecessors. The final level of classification, into divisions, is new and makes it possible to combine divisions across higher-level categories so that, for example, total incidence for leukemias and lymphomas of specific cell types can be derived. ICCC-3 includes all neoplasms of malignant behavior. Nonmalignant intracranial and intraspinal tumors are also included since there is relatively little difference in presenting symptoms, treatment, prognosis, and late effects between malignant and nonmalignant tumors compared with those of other sites. Cancer registries are increasingly collecting data on nonmalignant central nervous system (CNS) tumors and including them in routine statistics. This is especially important for childhood tumors because the single most frequent childhood CNS tumor, pilocytic astrocytoma, was downgraded to nonmalignant behavior in ICD-O-3.
Incidence
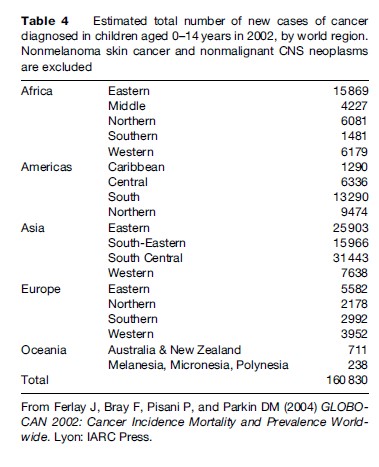
Tables 2 and 3 show estimated age-specific and agestandardized incidence rates during the 1980s and 1990s for cancer among boys and girls aged under 15 years in populations from different regions of the world. The age-standardized incidence of all cancers combined is typically between 70 and 160 per million children per year, corresponding to a risk of 1 in 1000 to 1 in 400 that a child will be affected during the first 15 years of life. Incidence tends to be relatively low in less developed countries of Asia, perhaps reflecting not only lower risk but also underdiagnosis and underascertainment. The results for Africa are probably also affected by underregistration, but in some sub-Saharan countries this is offset by very high risk for a few types of cancer, resulting in total rates that are comparable with those in Europe and the Americas. Table 4 shows estimates of the total numbers of cases of childhood cancer diagnosed in each world region in 2002. Worldwide, there were an estimated 161 000 new cases, one-half of which were in Asia.
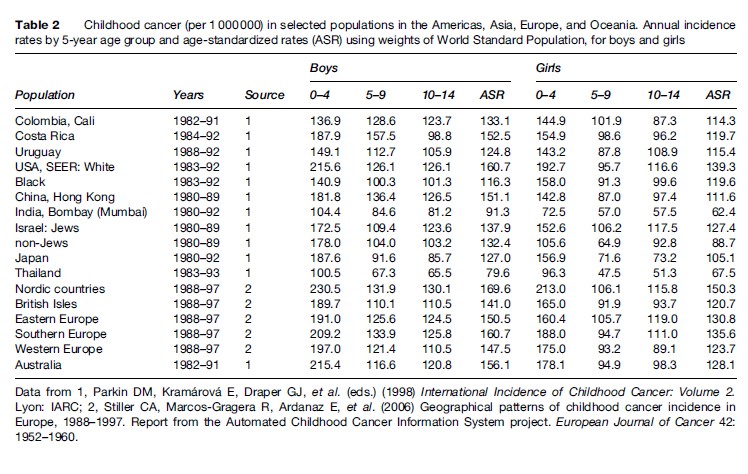
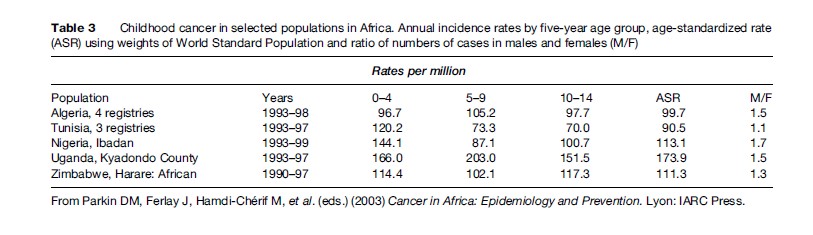
Incidence is usually highest in the first 5 years of life, falls to a minimum around age 9–10 years, and rises again thereafter. Boys are affected more often than girls. There tends to be a larger proportion of boys among cases in developing countries, maybe as a consequence of selective underregistration of girls. The male excess is greatest for lymphomas and less marked for leukemia, CNS tumors, neuroblastoma, and soft-tissue sarcomas. Retinoblastoma and renal tumors occur with similar frequency among boys and girls. Girls have a higher incidence than boys for melanoma and certain germ cell tumors and carcinomas.
While total incidence varies only moderately between world regions, there is more marked variation for diagnostic groups and subgroups. Figures 1–4 show the contribution of the 12 main diagnostic groups in the same populations as those in Tables 2 and 3. Because ICCC-3 was introduced very recently, the data are grouped according to the previous ICCC (Krama´rova´ and Stiller, 1996), which was defined in terms of ICD-O-2. As mentioned above, the diagnostic groups are comparable between the two classifications although the terminology has changed. Figure 5 shows incidence among Hispanic (Latino) and non-Hispanic children in selected areas covering more than 80% of the United States during 1999–2003; these data are grouped according to ICCC-3.
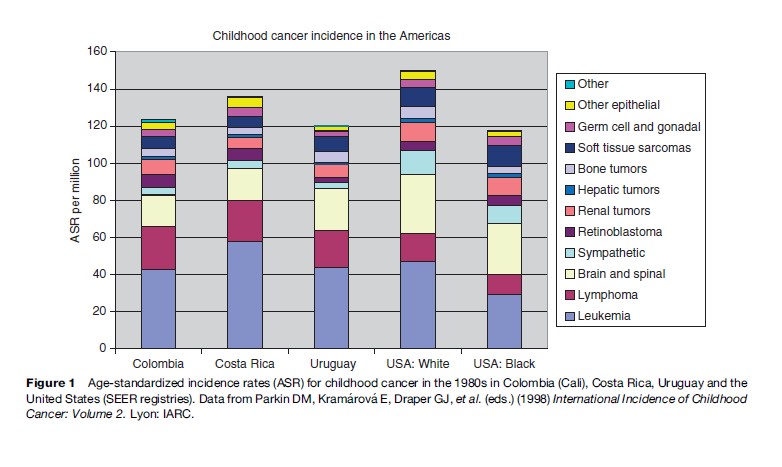
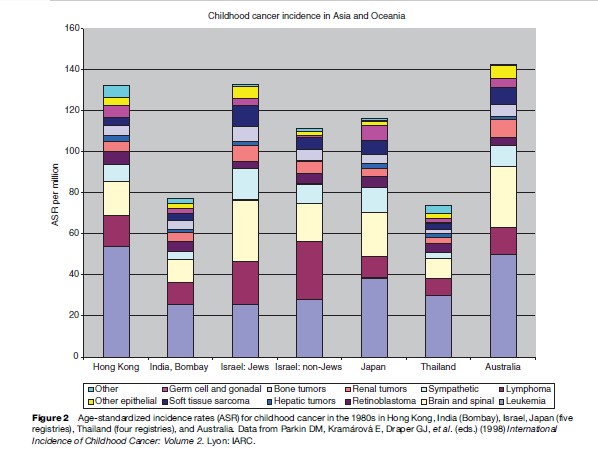
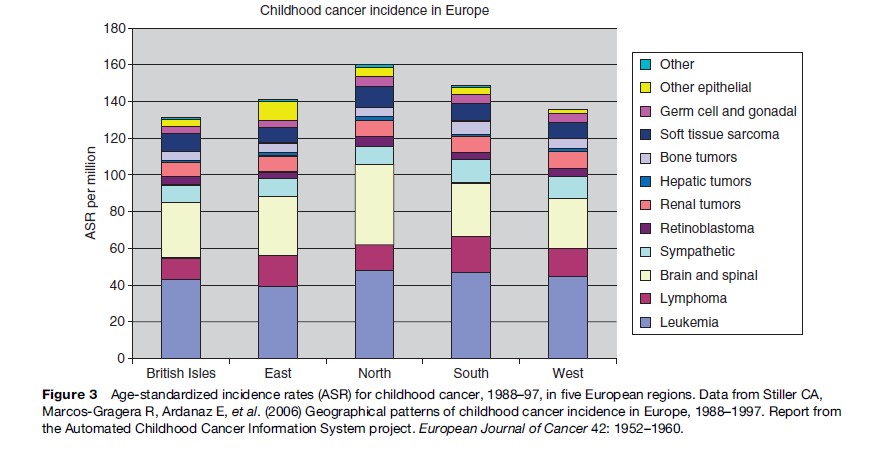
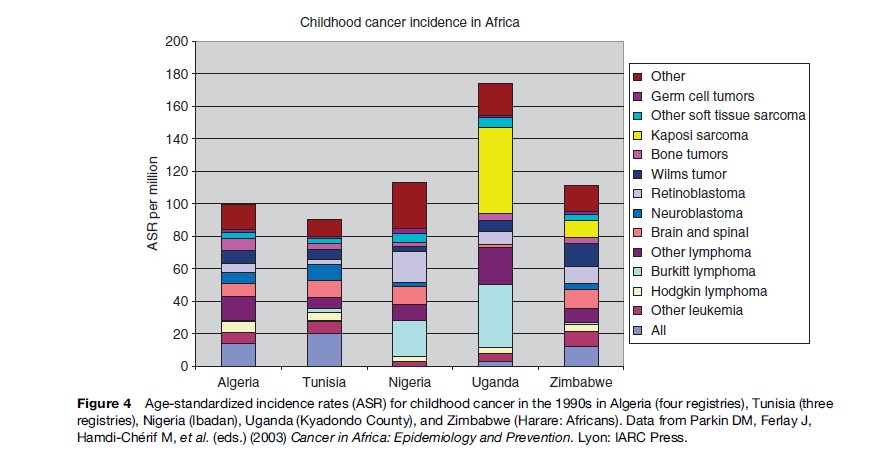
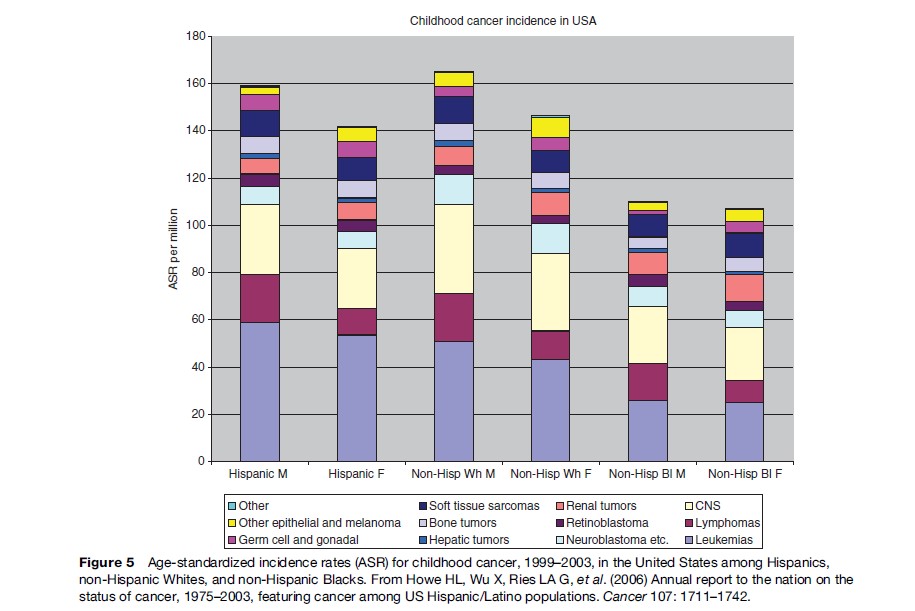
Among children in the Americas, Europe, and the industrialized countries of East Asia and Oceania, leukemias form the largest diagnostic group, with an age-standardized rate (ASR) of 40–50 per million and often accounting for about one-third of all childhood cancers. The highest rates have been recorded among the Hispanic populations of the United States and Costa Rica, and in Hong Kong. Leukemia has somewhat lower incidence elsewhere in Asia, in North Africa, and among black children in the United States, but is still the largest diagnostic group. In much of sub-Saharan Africa, incidence is yet lower and leukemias are outnumbered by some other diagnostic groups. These variations reflect the more pronounced variations in acute lymphoblastic leukemia (ALL), which comprises around 80% of childhood leukemia in the most affluent populations, 60–70% in many developing countries of Asia and among U.S. Blacks, and seldom more than 50% in sub-Saharan Africa. The highest incidence occurs around the ages of 2–4 years, and much of the variation in total incidence is accounted for by the size of this early childhood peak. Incidence of ALL in Eastern Europe used to be substantially lower than in the West, but it has increased over recent decades and the early peak has become correspondingly more pronounced (Steliarova-Foucher et al., 2004). In contrast, the deficit of ALL among black children in the United States compared with Whites is most marked in early childhood, and the peak is even more attenuated in those countries with the lowest total rates (Parkin et al., 1998). Most other cases of childhood leukemia are acute myeloid leukemia (AML), whose incidence shows little international variation.
Lymphomas have an ASR of 10–20 per million and account for around 10% of childhood cancers in the industrialized countries of North America, Europe, East Asia, and Oceania; the incidence and relative frequency are somewhat higher in the South of Europe than elsewhere in the continent. In much of Latin America, South and West Asia, and North Africa, lymphomas represent 15–25%; in parts of the Middle East and North Africa, they slightly outnumber leukemias. The highest incidence is found in much of tropical Africa, where lymphomas can have an ASR of 30–60 per million children and account for 30–40% of all childhood cancers. Lymphomas also account for more than one-third of all childhood cancers in Papua New Guinea, though incidence cannot be reliably estimated (Parkin et al., 1998). In the past, Burkitt lymphoma was the most common type of childhood cancer in these regions of high lymphoma incidence, though in some places it has been overtaken by Kaposi sarcoma; there are also substantial numbers of cases of other types of non-Hodgkin lymphomas (NHL), but Hodgkin lymphoma is relatively rare. Almost everywhere else in the world, NHL (including Burkitt lymphoma) accounts for most cases of childhood lymphoma, but Hodgkin lymphoma is also relatively frequent. However, Hodgkin lymphoma is more common than NHL among children of Eastern Europe and some Hispanic populations in the Americas, and it is rare in East Asia. In developing countries, the incidence of Hodgkin lymphoma rises gently through childhood, sometimes even reaching a peak at age 5–9 years, whereas in more affluent countries the incidence is low until the onset of adolescence and then rises much more steeply.
Brain and spinal tumors are second in frequency only to leukemias in industrialized countries, where they account for 20–25% of all childhood cancers. Rates are higher in countries where there is more systematic registration of nonmalignant tumors, which can account for 13–18% of all CNS neoplasms. Recorded incidence is lower in less developed countries, at least in part because of underdiagnosis. The most frequent subtypes are astrocytomas and other gliomas, embryonal tumors including medulloblastomas and primitive neuroectodermal tumors, and ependymomas.
Neuroblastoma has an ASR of 10–13 per million in most industrialized countries and accounts for 6–9% of childhood cancer. Incidence is lower in developing countries, probably because of underdiagnosis, but consistently very low rates in tropical Africa suggest that there the risk may also be lower (Parkin et al., 2003). Neuroblastoma is predominantly a disease of very young children, and is the most common cancer in the first year of life. Especially high incidence rates have been recorded in Japan, where until recently there was mass screening of infants for neuroblastoma, but this has now ceased. The effect of this practice on population incidence and mortality rates is discussed below in the section titled ‘Screening.’
Retinoblastoma usually has an ASR of 3–5 per million, but rates as high as 20 per million have been recorded in some developing countries. The international variation is largely accounted for by unilateral cases, whereas bilateral retinoblastoma has relatively constant incidence.
Malignant renal tumors are mostly nephroblastoma (Wilms tumor). This tumor was formerly believed to have constant incidence worldwide, but it is now clear that incidence varies considerably with ethnicity, being highest (9–12 per million) among black populations and lowest (often under 5 per million) among those of Asian origin.
Malignant hepatic tumors are less frequent. The two main types are hepatoblastoma, which occurs predominantly in infancy, and hepatocellular carcinoma (HCC), seen mainly in older children. Hepatoblastoma has fairly constant incidence worldwide, whereas HCC tends to be more common among children in East Asia, Melanesia, and sub-Saharan Africa, where it also has higher incidence among adults.
The incidence of malignant bone tumors increases with age during childhood. The two main types are osteosarcoma, with fairly constant incidence worldwide, and the Ewing sarcoma family of tumors, which are notably rare in black and East Asian populations.
The highest incidence of soft tissue sarcomas in childhood is found in parts of East and Central Africa with high levels of human herpesvirus 8 (HHV8) infection, where most cases are Kaposi sarcoma. Incidence rose dramatically in these countries with the worsening of the AIDS epidemic, exceeding 50 per million children in Kampala, Uganda (Parkin et al., 2003). In other parts of the world, incidence of childhood soft tissue sarcomas rarely exceeds 6 per million and rhabdomyosarcoma is the most frequent type.
Germ-cell tumors usually constitute less than 4% of all childhood cancers. The most frequent primary sites are gonadal and intracranial. The incidence is highest in East Asia.
Carcinomas and melanomas account for well under 5% of all childhood cancer. The most common site for carcinomas is the thyroid, but the incidence seldom exceeds 1.5 per million. The exception is the dramatically raised rates seen in areas of Belarus, Russia, and Ukraine that were most contaminated with radioactive iodine from Chernobyl. In North Africa, carcinoma of the nasopharynx is more frequent among children than thyroid carcinoma, and incidence of up to 3 per million has been recorded. The incidence of melanoma among children is highest in Australia, which also has very high incidence among adults.
The spectacular, and probably temporary, increases in the incidence of Kaposi sarcoma in some sub-Saharan African countries and of thyroid carcinoma in part of Eastern Europe have already been mentioned. Otherwise, changes over time in the recorded incidence of childhood cancer have been relatively small, though statistically significant in large data sets. Incidence rose at a rate of 1.0% per annum between the 1970s and 1990s in Europe (Steliarova-Foucher et al., 2004), and at 0.6% per annum between 1975 and 2003 in the United States. Some of the increase, at least for certain diagnostic groups, is undoubtedly artifactual. In particular, part of the increase in childhood CNS tumors can probably be attributed to the introduction and diffusion of more sensitive methods of diagnosis, at least where the increases have been most pronounced for low-grade tumors that can have a long natural history. Increases in the incidence of neuroblastoma among infants are likely to be due in part to increased detection rates for asymptomatic tumors, which can regress spontaneously. The effect of population screening on incidence rates is discussed in the section on screening below. Changes in incidence resulting from changing exposure to known exogenous risk factors are discussed in the sections titled ‘Etiology’ and ‘Prevention’ below. Improved ascertainment by cancer registries probably also accounts for some of the increases in incidence. The observed trends have not, however, been uniform across diagnostic groups, nor for children of all ages within diagnostic groups. Therefore, it seems likely that changes in incidence also to some extent reflect changes in risk. Some well-known patterns of variation in childhood cancer occurrence between populations are widely accepted as real, and there seems to be no reason why real temporal variations should not also exist.
Etiology
The causes of the great majority of cases of childhood cancer are unknown, especially in industrialized countries. Known risk factors may be broadly grouped as (a) genetic, (b) environmental or exogenous, and (c) other factors that could be genetic or environmental in origin.
In 1991, it was estimated that 3% of childhood cancer in the British population was associated with known genetic disease. Among mendelian disorders, the largest contributions were from heritable retinoblastoma, neurofibromatosis type 1 (NF1), and tuberous sclerosis. Retinoblastoma is the classic example of a genetically determined cancer (Lohmann and Gallie, 2004). It arises in the retina when there are two mutations of the RB1 tumor suppressor gene. In nonheritable cases both mutations are postzygotic, but in heritable cases the first mutation is prezygotic, either as a new germ cell mutation or inherited from a carrier parent. Heritable retinoblastoma is autosomal dominant with about 90% penetrance, hence the offspring of survivors have a 45% chance of developing the disease.
Children with NF1 have a relative risk for CNS tumors and soft tissue sarcomas of around 40; there is a 4–5% risk of developing optic nerve glioma, the most frequent CNS tumor in children with NF1. Children with tuberous sclerosis have a 5–15% risk of developing a CNS tumor. The estimate of 3% as the heritable fraction of childhood cancer did not include Li-Fraumeni syndrome, which is defined by specific patterns of familial aggregation of cancers and is usually caused by germline mutations of the tumor suppressor gene TP53 (Varley, 2003). Germline mutations of TP53 are present in most children with adrenocortical carcinoma and perhaps 10% of cases of childhood rhabdomyosarcoma and 3% of childhood osteosarcoma, suggesting that they could account for about 0.6% of all childhood cancer. Numerous other hereditary syndromes predispose to various childhood cancers (Garber and Offit, 2005), but the numbers of attributable cases are relatively small. Overall, the risk of childhood cancer for siblings of children with cancer is double that in the general population. Virtually all of the excess can be accounted for by known hereditary syndromes.
A wide range of constitutional chromosomal abnormalities together account for about 1% of all childhood cancers (Stiller, 2005). The largest contribution is from the excess of leukemia in children with Down syndrome.
Countless environmental exposures have been suggested as risk factors for childhood cancer, but for most of them the evidence is inconclusive or they can only account for a minute proportion of the total incidence. One of the very few firmly established risk factors is ionizing radiation. The raised risk of cancer among children irradiated in utero was discovered in the 1950s, but obstetric irradiation accounts for hardly any cases of childhood cancer today, since its almost total replacement by ultrasound. X-ray treatment for benign childhood conditions including tinea capitis has long been known to be carcinogenic (Ron, 2003). This practice is also obsolete and virtually no current cases of childhood cancer can be attributed to it. Radiotherapy for cancer can give rise to subsequent malignancies but most of these occur after childhood. The explosion at the Chernobyl nuclear plant in 1986 resulted in large numbers of thyroid cancers among children in areas most heavily contaminated by fallout, especially radioactive iodine, but there is little evidence of an excess in less heavily exposed regions. Excesses of childhood leukemia in the vicinity of certain nuclear reprocessing plants seem most likely to be attributable to factors other than radiation (Laurier et al., 2002). There is no general excess of cancer among children living near to nuclear power stations (Laurier et al., 2002). Given the known carcinogenicity of ionizing radiation, it is plausible that some childhood cancers are attributable to naturally occurring radon and gamma rays, but risk estimates vary considerably and these exposures can only account for small numbers of cases (Laurier et al., 2001).
Ultraviolet radiation from sunlight is a proven risk factor for malignant melanoma in adults, and is presumably also responsible for some childhood cases. Epidemiological studies have consistently found a raised risk of leukemia among children with the highest exposure to electromagnetic fields (EMF) from electric power cables, though the reasons for this remain controversial. Fewer than 5% of children are exposed to levels of EMF that have been associated with the raised risk, and EMF therefore could only account for a very small fraction of childhood leukemia (Kheifets et al., 2006). There is no consistent evidence that EMF exposure is associated with childhood CNS tumors or that residential proximity to radio frequency transmitters is a risk factor for childhood cancer.
A few specific infections are involved in the etiology of certain childhood cancers. Numerically, the most important are Burkitt lymphoma, Hodgkin lymphoma, nasopharyngeal carcinoma, HCC, and Kaposi sarcoma. Epstein-Barr virus (EBV) is implicated in virtually all cases of Burkitt lymphoma occurring in the tropical regions of high incidence, with endemic malaria as a cofactor whose precise role is not yet clear. Around 70% of childhood Hodgkin lymphoma and almost all cases of nasopharyngeal carcinoma can also be attributed to EBV. Most cases of HCC are attributable to hepatitis B infection, especially in regions where hepatitis B is most prevalent; the reduction in incidence following immunization against hepatitis B is discussed in the section titled ‘Prevention’ below. Kaposi sarcoma is attributable to HHV8. In regions of sub-Saharan Africa most severely affected by the AIDS epidemic, immunosuppression consequent to infection by HIV makes uncontrollable HHV8 infection more likely, resulting in increased incidence of Kaposi sarcoma. Controversy persists over the possible role of SV40 as an etiological agent for various cancers, of which NHL, CNS tumors and osteosarcoma are the most frequent among children. Epidemiological studies of large populations exposed to SV40 from contaminated polio vaccine have failed to show that it is a risk factor for these cancers.
A wealth of epidemiological evidence indicates that infection is sometimes involved in the etiology of childhood leukemia (McNally and Eden, 2004). Two hypotheses have been extensively investigated. Kinlen suggested that some childhood leukemia is a rare response to a specific but, as yet, unidentified infection and that higher incidence would be expected when groups of susceptible and infected people are brought together. This is supported by a series of studies that have found raised incidence of childhood leukemia in communities that experienced an unusually high degree of population mixing, often though not always as a result of migration (Kinlen, 2004). According to Greaves’s hypothesis, heightened immune response to delayed exposure to infection acts as a promoter of common ALL, the most frequent subtype in industrialized countries, in children who were born with a premalignant clone if their immune system is underdeveloped (Greaves, 2006). Epidemiological evidence supporting this hypothesis includes findings of reduced risk associated with breast feeding and with daycare attendance in very early childhood (Buffler et al., 2005). It should be emphasized that these two hypotheses are not mutually exclusive (McNally and Eden, 2004; Greaves, 2006).
Children who were given certain cytotoxic drugs for a first cancer have an increased risk of developing a second primary but, as with those induced by radiotherapy, the subsequent malignancies can only account for a tiny proportion of all childhood cancers. Diethylstilboestrol is an established trans placental carcinogen, causing clear cell adenocarcinoma of the vagina or cervix in offspring of some women whose mothers took it during the index pregnancy, but since this practice was discontinued in the 1970s it should not be responsible for any newly diagnosed childhood cancers. There was considerable public concern in the 1990s over the possibility that infants given intramuscular vitamin K to prevent hemorrhagic disease were at increased risk of leukemia and other cancers, but analyses of data from several large studies have found little evidence to support this hypothesis.
Case reports have raised the possibility of an excess of cancer in children born following assisted reproductive technology (ART), but follow-up studies of cohorts of children born after ART have not as yet found a significantly increased risk (Raimondi et al., 2005). As the number of children born after ART continues to increase, further studies are required since the overall picture of no difference in risk could conceal an increase (or decrease) associated with specific types of ART or causes of infertility.
A very large number of other environmental exposures have been proposed as risk factors for childhood cancer in general or for specific types. Such exposures could be experienced by the father preconceptionally, by the mother during pregnancy, or by the child, and could be dietary, domestic or, in the case of a parent, occupational. The most comprehensive review, covering all types of childhood cancer, remains that published by Little in 1999 (Little, 1999). More recent reviews have been devoted to particular diagnostic groups, notably leukemia and CNS tumors. The evidence on any specific agent is typically from a small number of studies, sometimes with limited consistency (Linet et al., 2005). A few associations have been found relatively consistently, and this may make them more credible. For example, benzene has been associated with childhood leukemia in several studies, either as a parental occupational exposure or through residence in localities believed to have an above average concentration, and these associations are given added plausibility by the established status of benzene as a risk factor for AML in adults (Buffler et al., 2005; Linet et al., 2005). There is little epidemiological evidence that air pollution from motor vehicle traffic increases the risk of childhood cancer, but methodological limitations of many studies and limited consistency between them mean that a small effect cannot be ruled out.
Most studies of domestic or occupational exposures depend on self-reported data that are subject to recall bias and for which there is little opportunity for independent validation. The evidence for many associations with occupational exposures, in particular, is rather limited. Relatively consistent associations have been found between paternal occupations related to motor vehicles, in chemical or petroleum industries, or occupations involving exposure to paints, and occurrence of leukemia or CNS tumors in their offspring. A consistent link has been found across several studies between Ewing sarcoma and parental occupation in farming, but it remains unclear whether this is attributable to exposure to agricultural chemicals, infectious diseases of farm animals, or some other factor
